Company Introduction
KVS Technologies and Automation having strong professional experience of CSV and regulatory services in Healthcare, Pharmaceuticals and Life sciences industries to meet the customer expectations. KVS is promoted by Shankar Sapavadiya. Promoters having 32+ Years experience in Instrumentation, Automation and Validation in Pharma Industries. Main area of interest is to provides CSV and compliance services to Life science, Healthcare and Pharmaceutical Industries of India and worldwide.
KVS Team having 18+ years of experience of Validation of computerised Systems, IT Systems. We build most effective environment for customers on Current updates of 21 CFR Part 11, EU Annex11 regulatory Compliance. KVS has provided CSV and regulatory compliance services to more than 150 companies in India and worldwide to life sciences, Pharmaceuticals, Chemicals, Medical Device, cosmetics.
Our Vision is to be the best partner in Validation of Automated computerised system and IT system and regulatory compliance services for healthcare, pharmaceuticals and life sciences industries.
Our Mission is to provide the most intuitive, Transparent, Economical, Effective CSV and regulatory compliance services to Healthcare, Pharmaceuticals and Life sciences industries as Current regulatory requirements.
LIMS
Laboratory Information Management System, is software that helps laboratories manage samples, data, workflows, and instruments, improving efficiency accuracy, and compliance
ERP
Enterprise Resource Planning (ERP) is a software system that helps businesses streamline their core processes. It can be used to manage finance, HR, manufacturing, supply chain, QC , QA ,sales, and procurement.
EBMR
eBMR stands for Electronic Batch Manufacturing Record, a digital system that tracks and manages the entire batch manufacturing process, replacing traditional paper-based records and enhancing efficiency, accuracy, and compliance.
SCADA
A SCADA (Supervisory Control and Data Acquisition) system is a combination of hardware and software used for industrial process automation, enabling real-time monitoring and control of equipment from a central location
DCS
A Distributed Control System or DCS is a computerized system that automates industrial equipment used in continuous and batch processes, while reducing the risk to people and the environment.
PLC
A PLC, or Programmable Logic Controller, is an industrial computer designed to automate and control various processes and machinery in manufacturing and other industries, replacing traditional relay-based systems with programmable logic.
EQMS
EQMS, or Electronic Quality Management System, is a software solution that helps organizations manage and automate their quality processes, ensuring compliance and improving overall quality
eCTD
eCTD stands for Electronic Common Technical Document, a standard format for submitting regulatory information, like applications and reports, to health authorities in a harmonized way.
What is Validation?
Validation is Establishing documented evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and quality attributes.
Benefits of Validation

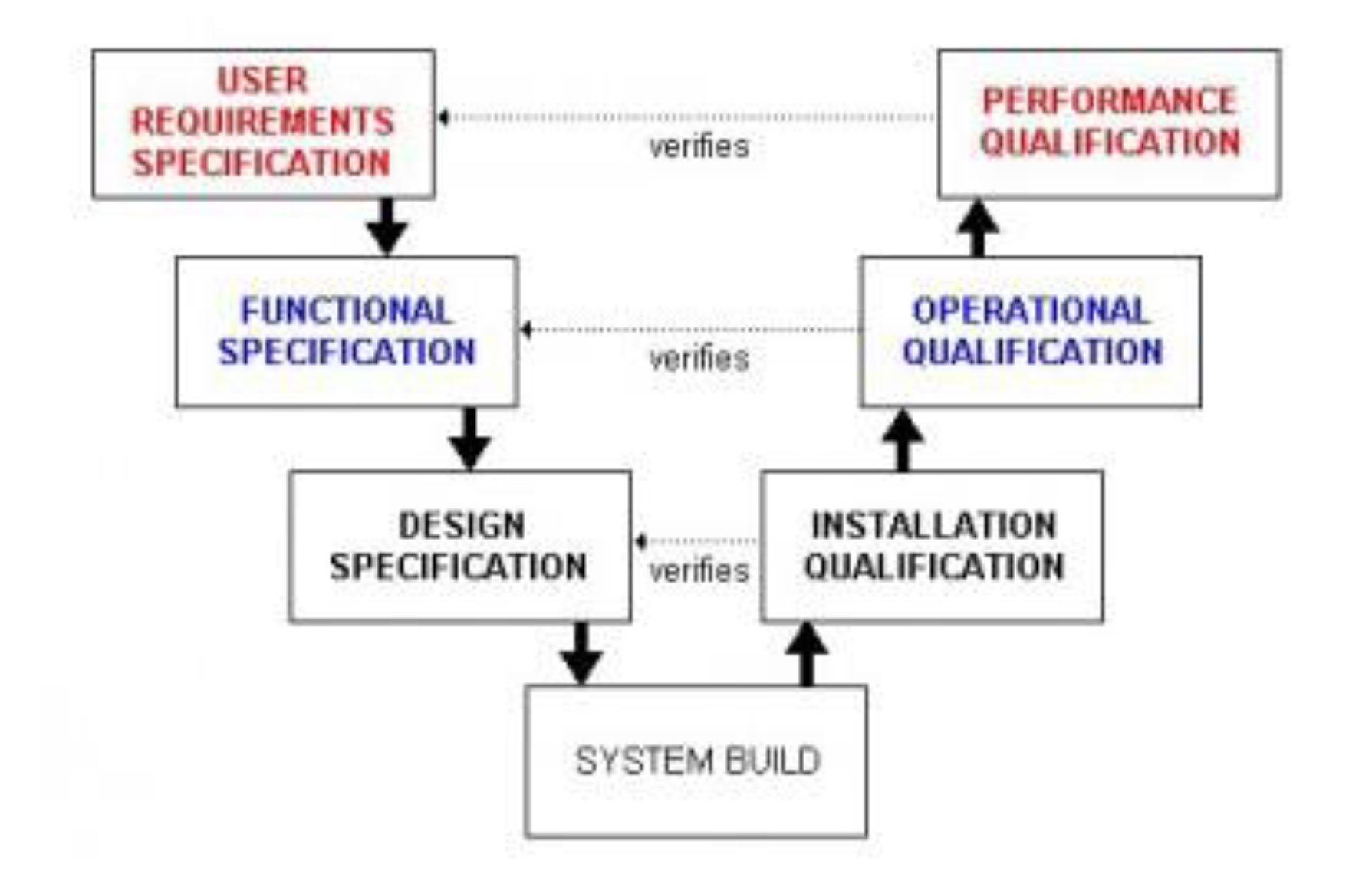
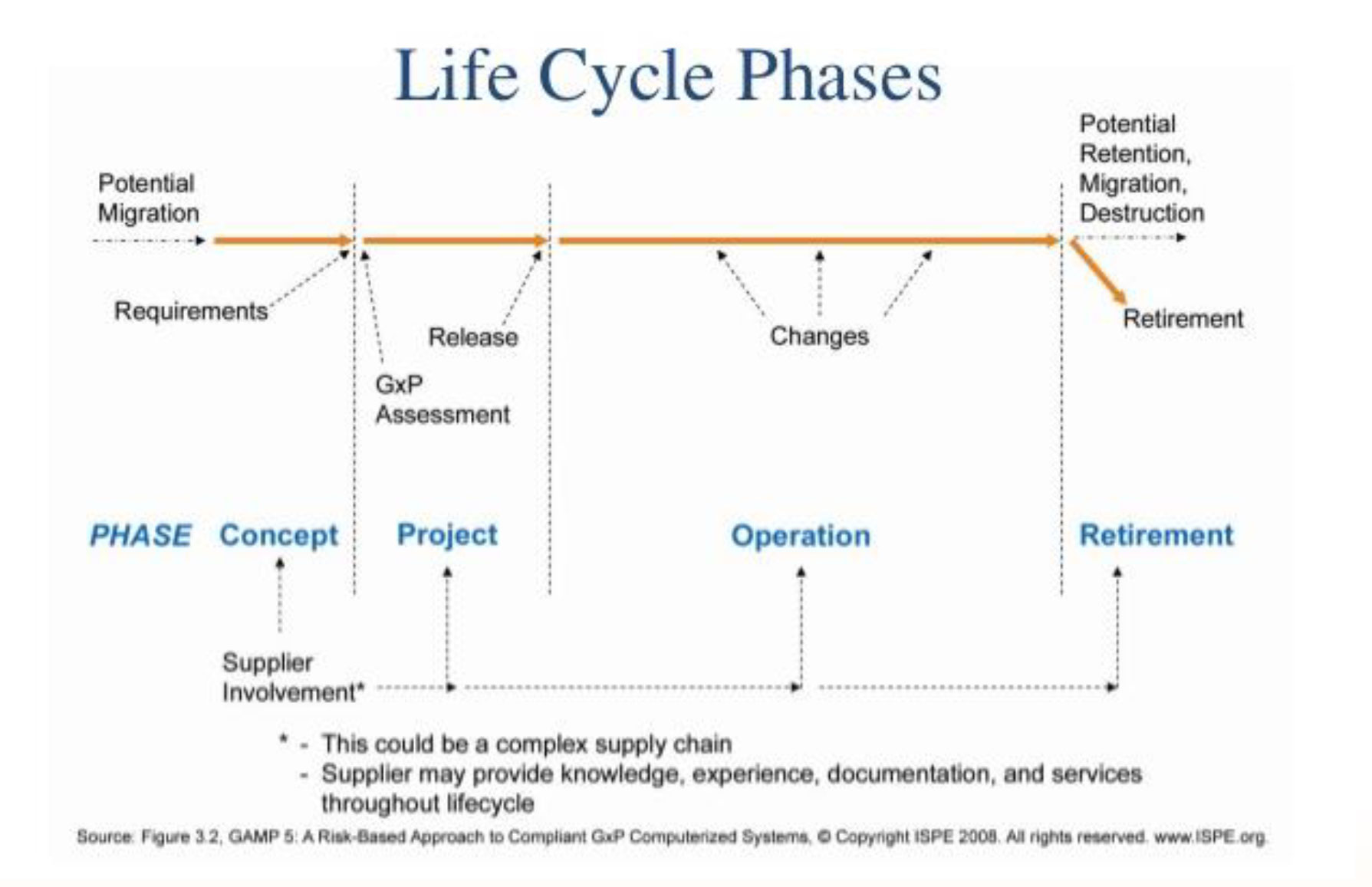
What is Computer System Validation?
Computers are widely used during Product development ,Product Testing, Analysis and Manufacturing. Proper functioning and performance of Automated System Software and Computer Systems play a major role, reliability and accuracy of product output. Computer System Validation is the process of documented evidence for verification of system functions and the performance is meeting to User Requirements Specifications, as well as data integrity and system maintenance. The written documentation must be in alignment with the Industry Standards. Therefore, Computer System Validation (CSV) is an essential part of any good development and manufacturing practice.
Benefits of ValidationBusiness Benefits of Validation
There are many major business and compliance benefits of qualification and validation.
Business Benefits of Validation
GxP Compliance
Meeting all applicable pharmaceutical and associated life-science regulatory requirements.
GxP Regulated Computerised Systems
Computerised systems that are subject to GXP regulations. The regulated company must ensure that such systems comply with the appropriate regulations.
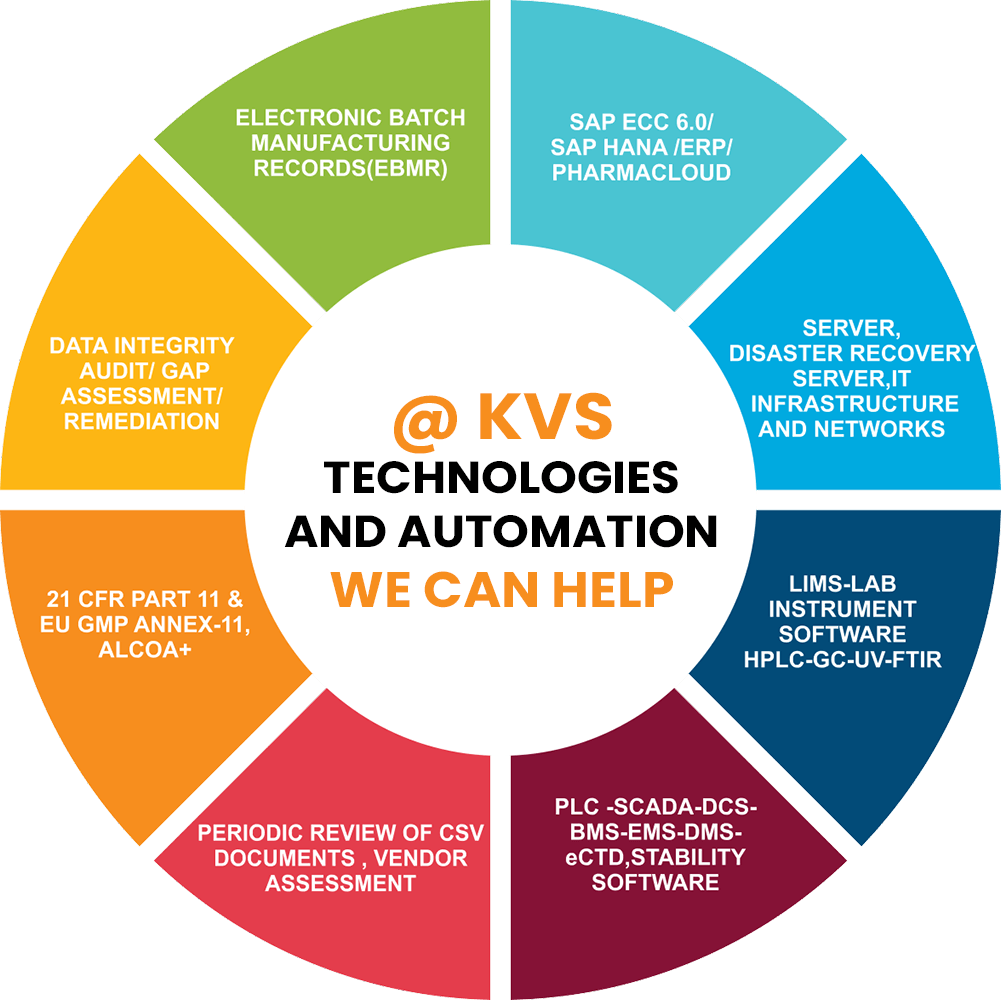
CSV Services Provided by KVS Technologies and Automation
As KVS Technologies and Automation we work as Your Validation Partner, we work with commitments. Our rooted knowledge with the industries, stems from our continuous learning, a key to our market leadership.
IT System/ ERP
• IT Infrastructure
• Server & Data centre
• SAP(ECC 6.0 )and HANA
• Pharma cloud ERP System
• E-BMR Software
• Document Management
System
• CAPA and Change Control
Management Software
• Training management
software
Lab Instruments
• HPLC, GC System
• Empower software
• Lab Solution software
• LIMS Validation
• Non- Chromatography
System
• Stability chamber
software
• Clinical research
software
Validation of System
• PLC- SCADA System
• DCS based System
• BMS – EMS System
• Track and Trace system
• Vision system
• Access control system
• Equipment Qualification
• API process plants
• Purified Water system
• Medical devices
Other Services
Our rooted knowledge with the industries, stems from our continuous learning, a key to our market leadership.
Services
--
- GAP Analysis of Software/System
- 21 CFR Part 11 and EU GMP Annex 11 Impact Assessment.
- Standard Operating Procedure Preparation and Review.
- Compliance Audit/Third Party IT Audit
- Vendor Assessment / Vendor Audit
- Periodic review of Computerised System.
- Deputing CSV /IT work force and Engineers team at site.
- Review of CSV documents, Change control, CAPA and other IT solutions.
Services
--
- Pharmaceuticals Industries
- Medical device
- OEM Machine Manufacturer
- Clinical Research Organisation
- Food Industries
- SAP and ERP implement partners
- IT Industries
- Healthcare and Hospital
Our Expert Team
Reduce your stress and strain and Increase your production and profitability. Meet your quality Automation and Validation Partner.



































